The heart is a truly amazing organ. To live, and maybe love, we need it to be functioning every day and every night of our lives. If something goes wrong with our heart, we are in trouble. Unfortunately the stresses on the heart are enormous, and not all hearts are infallible. A range of problems such as infections, diseases of the blood vessels, arrhythmia and congenital defects can affect the heart. Heart diseases are the leading cause of death in many countries, including the U.S., Canada, England and Japan, where they account for about a quarter of all deaths.
If the flow of blood to a section of the heart muscle is suddenly blocked, that part of the heart muscle is deprived of oxygen, its energy levels begin to fall, and it may cease to contract. This is a heart attack, which is also known as a myocardial infarction. If the blockage, which is normally a blood clot, is not removed in a timely manner, oxygen depravation (ischemia) may occur in all cardiac tissue downstream from the obstruction, leading to its cell death (infarction). The process is halted when oxygenated blood begins to flow back into the affected areas, which is called reperfusion. The amount of damage to the heart depends on the amount of time that has passed between the heart attack and reperfusion. The offending clot is typically removed by an angioplasty. Days and even weeks after a heart attack and reoxygenation, previously oxygen-starved myocardial (heart muscle) cells can die from reperfusion injury. The mammalian heart has little capacity to regenerate, and the injured myocardial cells are replaced with non-contractile scar tissue.
When Hina Chaudhry, associate professor of medicine at Mount Sinai School of Medicine, heard that women in the last few months of pregnancy have the highest rate of recovery from heart failure, she wondered whether it might be the fetal cardiomyocytes that are doing the repair. Using fluorescent proteins, Chaudhry has confirmed her suspicions that fetal stem cells cross the umbilical cord and are attracted to injured maternal hearts, where they are capable of differentiating into a variety of cell types, including beating cardiomyocytes. In her experiments Chaudhry used genetically modified male mice that express the green fluorescent protein from the jellyfish to impregnate normal virgin female mice, resulting in non-fluorescent female mice that are impregnated with fluorescent embryos. Under normal conditions no fluorescent heart cells were observed in pregnant and post-natal females. However, if the heart of the pregnant mother was injured, green fluorescent cardiomyocytes, smooth muscle and endothelial cells were observed in the injured areas of the heart. Clearly the fetus helps mend its mother's broken heart.
This is a stunning result. It implies that the heart sends out a chemical distress signal after a heart attack. In all heart-attack victims the "help, cardiomycytes in trouble, please send stem cells" signal futilely echoes through out the body, except in women in the late stages of pregnancy, where the signal arrives in the embryo, which responds by sending lifesaving embryonic stem cells. And these stem cells then nearly magically manage to find the source of the chemical distress signal, before differentiating into the required heart cells. It's very interesting, but can this knowledge be used to help the nearly 1 million people who have a heart attack in the U.S. every year?
Charles Murry from the Center of Cardiovascular Biology at the University of Washington certainly thinks so. He and his co-researchers have just published a paper in Nature that described how they injected billions of fluorescent human embryonic stem cells into three macaque monkeys two weeks after they had had a heart attack. Within three months the stem cells had differentiated into functional cardiomyocytes (heart muscle cells) that were fully integrated into the monkey hearts.
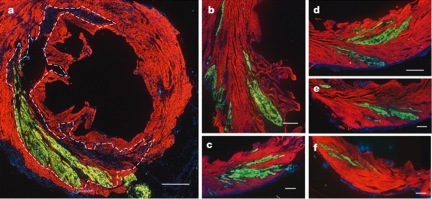
Confocal fluorescence microscope images of a macaque heart that has been subjected to a heart attack and injected with a billion human stem cells expressing green fluorescent protein. All the images show the human stem cells that have become green fluorescent heart muscle cells and have been incorporated into the monkey heart. The region enclosed within the dotted line was directly affected by the heart attack (infarction) and images (b-f) show the surrounding areas.
On average the damaged hearts restored 40 percent of their heart muscle, "which, if we could do it in a patient, would certainly make an impact on critical heart function," says Charles Murry.
The human stem cells were also genetically modified with GCaMP3, a specially modified protein from the jellyfish that only fluoresces in the presence of calcium. Since calcium triggers the heartbeat, the cells flash every time they beat. In the live macaques the fully functioning muscle cells derived from the human stem cells flashed in perfect synchrony with the electrocardiogram of the monkey heart, showing that the new cells were following the rhythm set by the macaques' body.
When asked about the first human trials, Murry said, "We have a road map in place, and we figure we can do our first patient in four years."