
By Joni Blecher
Think you can't live without a pulse? Not necessarily. Patients with heart failure no longer have to remain in a hospital waiting for a donor heart. A device that once kept patients alive only long enough to receive a transplant is changing the way people with heart failure live for extended periods of time.
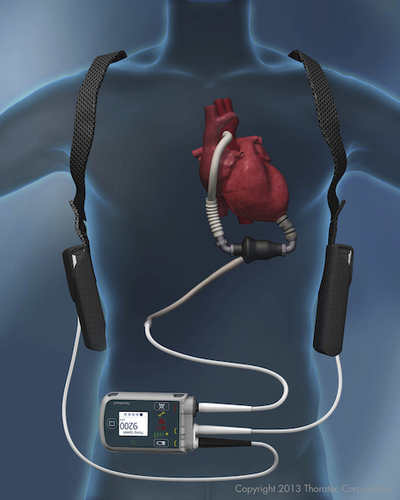 The left ventricular assist device (LVAD) has gone from being a bridge to a heart transplant to a long-term therapy option. An LVAD helps the heart pump blood to the rest of the body, thereby taking a lot of stress off of the heart. Surgically implanted, an LVAD pulls blood from the left ventricle into a pump and sends it to the aorta. The device is connected to a control unit and battery packs via a tube that runs outside of the body.
The left ventricular assist device (LVAD) has gone from being a bridge to a heart transplant to a long-term therapy option. An LVAD helps the heart pump blood to the rest of the body, thereby taking a lot of stress off of the heart. Surgically implanted, an LVAD pulls blood from the left ventricle into a pump and sends it to the aorta. The device is connected to a control unit and battery packs via a tube that runs outside of the body.
Traditionally, an LVAD was only used for patients in a hospital setting and wasn't intended as a long-term solution. Now, the devices are portable and can be managed at home. Since an LVAD increases blood flow, organs and muscles have more oxygen, making it easier for patients to exercise and become stronger. That way, if a donor heart does become available, they would potentially have better transplant outcomes.
According to the U.S. Department of Health and Human Services, every 10 minutes, someone is added to the national transplant waiting list. There are more people in need than organs available. Currently, there are thousands of patients living with LVAD implants worldwide. Devices are designed to last approximately 10 years, and there are patients that have been successfully living with them for seven years or more. One of the most famous recipients of an LVAD was Dick Cheney. The device kept the former vice president alive for 20 months until he received a heart transplant at age 71.
There is one major side effect for LVAD recipients: many lack a strong pulse, which can be problematic if someone attempts to administer CPR since it can damage the device.
Critically ill heart patients aren't the only ones benefiting from technological breakthroughs. Researchers at Draper Laboratory have developed an artificial lung technology that could rapidly transfer oxygen into the blood in a way that prevents clotting.
Currently, patients suffering from lung issues such as Chronic Obstructive Pulmonary Disease (COPD) and Acute Respiratory Distress Syndrome (ARDS) need to rely on mechanical ventilation or a treatment called extracorporeal membrane oxygenation. The latter draws blood from a patient and runs it through a device that removes carbon dioxide and adds oxygen before returning it to the body--often creating blood clots along the way. Both methods are hard on the body and are typically a life-saving measure.
The Draper Laboratory solution is a microfluidic oxygenator that resembles the lungs circulatory system. "Microfluidic technologies have shown promise for small-scale lab-on-a-chip applications, but the Draper project shows for the first time how microfluidic devices can be scaled for high blood flows required for acute interventions and chronic support," said Jeff Borenstein, Draper Laboratory's principal investigator for the project, which is funded by the National Institutes of Health's National Heart, Lung and Blood Institute.
Eventually, the goal is to use the technology in artificial lung devices that could be worn or carried by people with lung diseases. It's currently being tested with animal blood to make sure the oxygenation is working correctly and doesn't cause clots. Preliminary results indicate that it could scale for human clinical use. With about 150,000 cases of ARDS being reported per year in the U.S., this device could potentially change the way those people are treated and how they live over the long haul.
Visit XPRIZE at xprize.org; follow us on Facebook, Twitter and Google+; and get our newsletter to stay informed.
Joni Blecher is a freelance writer who has spent her career covering tech and a myriad of lifestyle topics. When she's not writing, you can find her exploring the food scene in Portland, OR.