A few months ago, I wrote a post about my early experiences as a young teacher 50 years ago. I learned a very important lesson on the very first day of my new job. It transformed the way I taught.
I was about to teach the eighth grade a unit on "Modern Atomic Theory." (Do they still teach it?) Somewhere in my training echoed the phrase "make it relevant." So I walked into my first class armed with a Fabulous Factoid that I hoped would forever endear me to my students. After describing the nucleus of the atom with its protons and neutrons and its orbiting electrons as a miniature solar system, the stage was set for the delivery of my FF.
"Want to know what makes up most of an atom?" I asked my class rhetorically.
"It's mostly empty space. In fact, if you took out all the space of all the atoms in a fleet of battle ships (maybe 17 ships) and packed all those subatomic particles next to each other, you would have a mass the size of a basketball that weighed as much as the entire fleet!"
I paused, satisfied that I had delivered my FF with sufficient drama. Silence greeted me. Then, at the back of the room, a boy slowly raised his hand. (I still remember his name). "Mrs. Cobb," asked David incredulously, "how do they know that?" I was stunned. I didn't know the answer. I figured I'd better not fake it. "Good question, David. I'll find out," I stammered.
Needless to say, the answer was not in the textbook (nor, for that matter was the FF). So I went to the library and found a children's book called The Story of the Atom. I noticed that the door to my classroom was closed and no one was watching me. So I taught the unit from this book and ignored the textbook. Each day I told a different story giving kids notes they could study from--How Henri Becquerel accidentally discovered invisible rays from a rock; rays that passed through opaque paper. How his friend, Marie Curie, measured the strength of these rays and discovered two more radioactive elements. 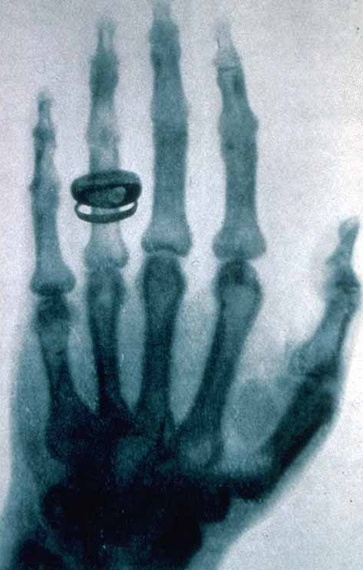
I described Roentgen's astonishment the first time he used his invisible X-rays to see the bones in his wife's hand. I told them how Ernest Rutherford shot some of Becquerel's rays, like bullets, through gold foil as if it wasn't there, except for an occasional deflection; (his experiments were the evidence for the enormous space in the atom and its nuclear structure), and how J.J. Thomson measured the mass of the tiny electron. Together, my students and I learned how Niels Bohr synthesized the evidence from many scientists to create a model of the atom (modern atomic theory) that explains chemical bonding, changes of state, the periodic table, the gas laws and more.
I am currently preparing a keynote speech for a STEM conference that will include this anecdote. Recollecting that moment, so long ago, impelled me to use the web to track down that book, The Story of the Atom, which had I found so useful. I found a book with that title by Mae Freeman and ordered it. But it included nothing of the history of science. Then I ordered another book by Laura Fermi, the widow of Enrico Fermi, a major player in the development of the atomic bomb. The title was slightly different: The Story of Atomic Energy, but hey, I could have remembered incorrectly. It was written for fifth graders but had no pictures, except for a section of photos in the middle of the book, and was 178 pages long! Wow! If this was a kids' book, it sure was a lot more challenging than stuff today's fifth graders are reading. It did have the information I had taught and I think I had read it. But the book I recalled using was heavily illustrated throughout (though only in two colors, possibly three). I'll keep looking until I find it.
What has impressed me about these early books was what children were supposed to read then and now. They had no "production value." Today's children's nonfiction are colorful throughout. The texts are broken up into smaller pieces interspersed with extraordinary art and photos. Publishers expect readers to have a certain amount of graphical literacy (read Myra Zarnowski's post if you don't know what this means). The tone of Mae Freeman's book was somewhat patronizing; Laura Fermi's was wordy. I don't think today's kids would have the patience to read what my students were expected to understand. Nevertheless, those old books were a step up from the text books, which were a nightmare of pedantry.
But my other take-away is that lesson from David I learned so long ago. It is important to really listen to students, especially when they ask a good question. It is important to recognize a good question when you hear it. In fact, what we need now more than ever is to keep listening for and asking good questions.
That's exactly what all those atomic scientists did.

